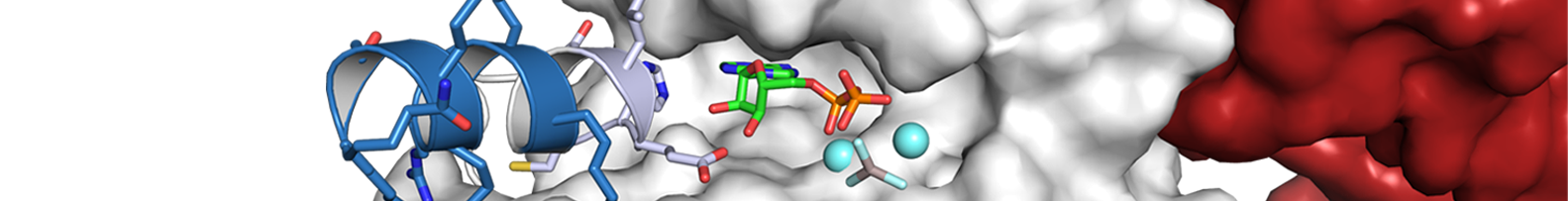
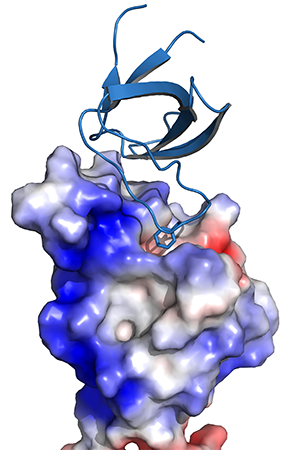
 The Nef protein of human (HIV) and simian (SIV) immunodeficiency viruses is an important replication and pathogenicity factor. Individuals infected with nef-deficient HIV-1 strains show very low viral loads and become long-term survivors without antiretroviral therapy. Nef-mediated internalization of CD4 from the surface of infected cells is highly conserved among primate lentiviruses and thought to promote the release of fully infectious viral particles and to prevent super-infection. The Nef proteins of HIV-2 and most SIVs also efficiently internalize CD3, an essential cofactor in T cell receptor. This function was entirely lost by HIV-1 and its simian precursors. Nef-mediated internalization of CD3 impairs the responsiveness of virally infected T cells to stimulation and may suppress the hyper-activation of the immune system that drives progression to AIDS. Nef stimulates clathrin-mediated endocytosis of CD3, CD4 and other cellular receptors by acting as an adaptor between endocytic sorting motifs and the assembly polypeptide complexes (AP-1, AP-2). Nef is targeted to the plasma membrane via an N-terminal myristoylation signal. We are interested in the structure and function of Nef and the multiple binding proteins that mediate the pathogenic function of Nef.
The Nef protein of human (HIV) and simian (SIV) immunodeficiency viruses is an important replication and pathogenicity factor. Individuals infected with nef-deficient HIV-1 strains show very low viral loads and become long-term survivors without antiretroviral therapy. Nef-mediated internalization of CD4 from the surface of infected cells is highly conserved among primate lentiviruses and thought to promote the release of fully infectious viral particles and to prevent super-infection. The Nef proteins of HIV-2 and most SIVs also efficiently internalize CD3, an essential cofactor in T cell receptor. This function was entirely lost by HIV-1 and its simian precursors. Nef-mediated internalization of CD3 impairs the responsiveness of virally infected T cells to stimulation and may suppress the hyper-activation of the immune system that drives progression to AIDS. Nef stimulates clathrin-mediated endocytosis of CD3, CD4 and other cellular receptors by acting as an adaptor between endocytic sorting motifs and the assembly polypeptide complexes (AP-1, AP-2). Nef is targeted to the plasma membrane via an N-terminal myristoylation signal. We are interested in the structure and function of Nef and the multiple binding proteins that mediate the pathogenic function of Nef.